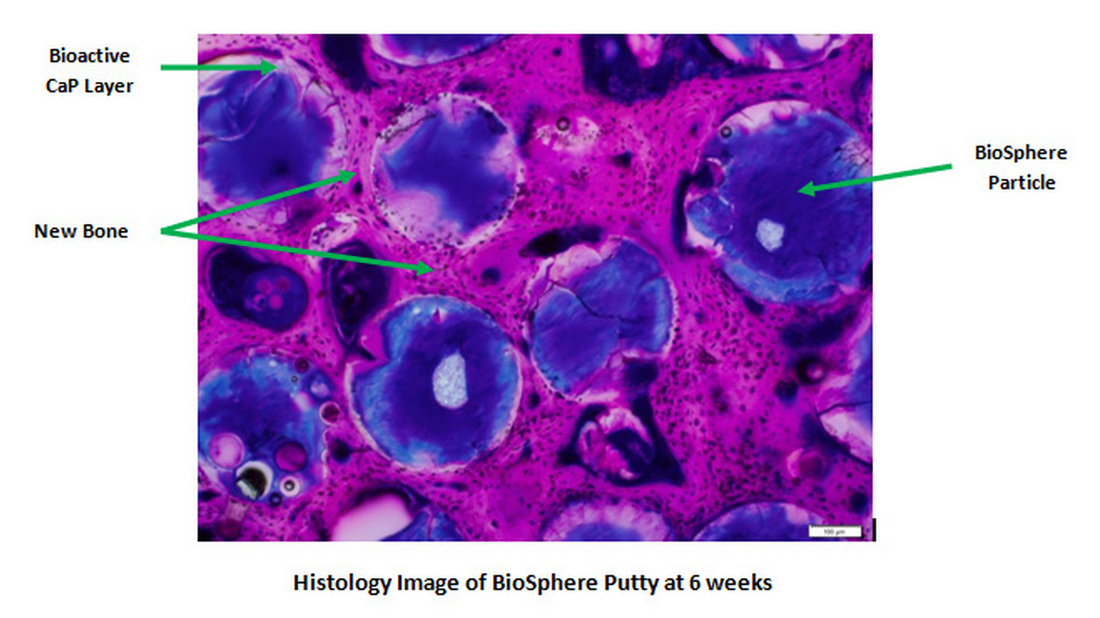
In Vivo Bone Healing
Data Driven Performance - In Vivo Study Results
- Bioactive glass has typically been used in a particulate form consisting of irregular particles and a broad size range (32-710 um).
- Instead of creating a bone graft with these "off-the-shelf" particles, Synergy utilized a unique spherical particle shape and conducted an in vivo optimization study to determine the size range that provided the best bone healing.
- Testing in a rabbit bone defect model showed that the Putty composed of 10:90 mix of 90-180 um spheres and 355-500 um spheres provided the best healing.
|
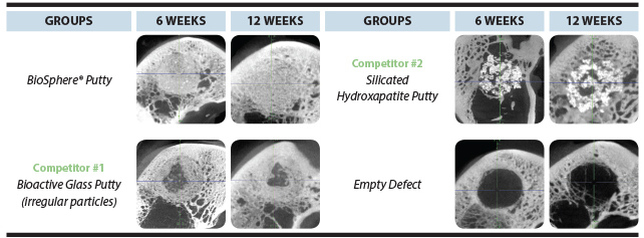
MicroCT
|
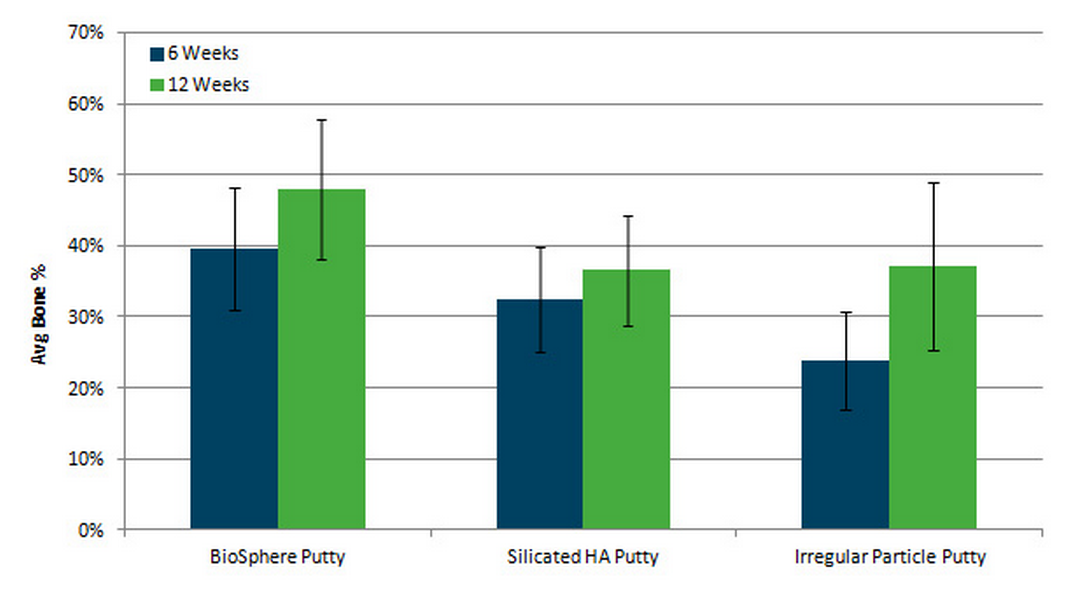
Faster bone growth throughout
the entire implant site* * ”In Vivo Evaluation of BioSphere® Bioactive Bone Graft Putty: Improved Bone Formation”, white paper on file |